Clinical Trials Explained
Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes.
People volunteer to take part in clinical trials to test medical interventions including drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioural treatments and preventive care.
Clinical trials are carefully designed, reviewed and completed, and need to be approved before they can start. People of all ages can take part in clinical trials, including children.
Clinical trials are designed in several different ways. The number of patients involved in a trial for cancer treatment, for instance, will often be lower than other types of trials.
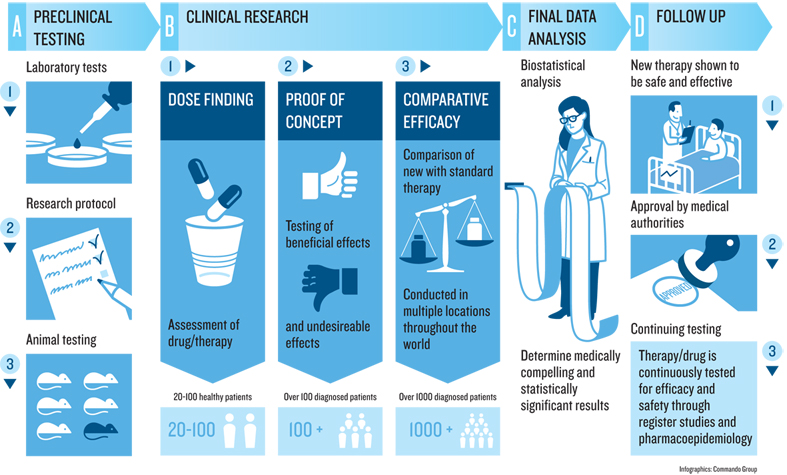
The illustration shows the progression of a clinical trial.